[Orthopedic Arthroscopy] Arthroscopic Autologous Osteocartilage Transplantation
Release time: 16 Dec 2025 Author:Shrek
Arthroscopic autologous osteochondral grafting is one of many treatments for cartilage lesions. Osteochondral grafting involves transplanting a healthy graft containing articular cartilage, cartilaginous landmarks, and subchondral bone to a sized area matching the lesion. Advantages of this technique include using hyaline cartilage instead of fibrocartilage to repair defects and maintain joint height and shape. Arthroscopic autologous osteochondral grafting is a single, inexpensive procedure that can even be performed on an outpatient basis. However, the procedure is technically challenging, and due to limitations in sourcing materials, it cannot completely treat large cartilage defects.
Indications and Contraindications
Indications for autologous cartilage grafting include independent, full-thickness cartilage defects with a diameter of 1–2.5 cm. Large defects (diameter greater than 2.5 cm) show poorer outcomes. Furthermore, this technique is generally limited to cartilage lesions with subchondral bone loss not exceeding 6 mm in depth. Autologous cartilage grafting is not suitable for knees with damage to adjacent articular cartilage (equivalent to type IV tibial cartilage injury), multiple type IV cartilage injuries, or unstable or poorly aligned knees. Patients over 35 years of age may have reduced expected outcomes, and some authors believe this technique is not suitable for patients over 50 years of age. Other contraindications include a history of knee infection, intra-articular fracture, rheumatoid arthritis, and extensive degenerative arthritis. Meniscus tears and ligament instability are not absolute contraindications, but these conditions must be addressed during cartilage grafting. Autologous cartilage grafting is most commonly used for the femoral condyle, although there are reports of its use for tibial plateau, trochlea, and patellar lesions.
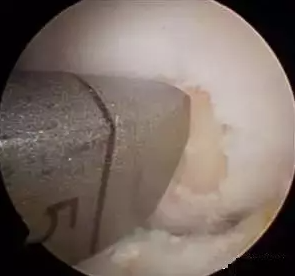
The osteochondral grafting (COR) system allows for precise harvesting of osteochondral emboli and implantation into a borehole of the same size as the defect area. A significant feature of the COR system is the cutting teeth on the harvesting cannula for more precise cutting depth, and a carefully designed drill bit for defect preparation. This drill bit makes it easier to align the recipient site hole perpendicular to the adjacent articular cartilage surface, resulting in a better match between the recipient and donor site tissue sizes.
Surgical Technique
First, a thorough arthroscopic exploration and evaluation of the knee is performed. When a localized, full-thickness cartilage defect is found, it is important to explore all areas of the knee joint, including the posterior recess and the submeniscal region, to identify and remove any mobile cartilage fragments. Arthroscopic grafting is suitable for most defect lesions; however, large and more posterior defects require extreme knee flexion to achieve an angle perpendicular to the articular cartilage, sometimes necessitating localized incision of the joint to achieve this angle. A lumbar puncture needle is used to determine the optimal angle of the approach, ensuring the approach is perpendicular to both the recipient and donor sites.
Arthroscopic autologous cartilage grafting is performed in four steps:
1. Assessment and preparation of the defect area;
2. Determination of the graft quantity;
3. Tissue harvesting;
4. Preparation of the implantation site and implantation of the autologous embolus.
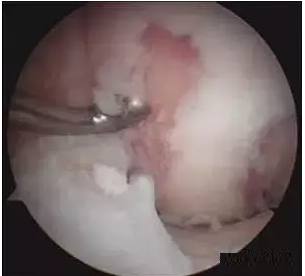
Assessment and Defect Preparation
The knee joint and defect area should be carefully assessed to ensure that selection criteria are met and that there are no contraindications for this surgery. Defect preparation involves removing all loose articular cartilage debris and creating a vertical cartilage wall at the defect margin using a curette or arthroscopic scalpel. Residual articular cartilage on the subchondral bone surface is removed, but extensive bleeding on the bone surface should be avoided. For better bone graft planning, the initial implanted emboli should be placed anteriorly at the edge of the articular cartilage immediately adjacent to the defect.
Determining the Number of Embedded Bone
Once the defect boundaries are determined, the number of grafts required can be estimated using a probe, or the size and depth of the defect can be measured using a harvesting cannula to determine which shape of emboli is best suited for the defect area. The depth of the defect can be estimated using the COR system, i.e., measured with a probe or a measuring ruler on the side of the harvesting cannula. Generally, a series of 6mm diameter graft emboli can be arthroscopically implanted and filled into the defect area. Larger emboli are available, but they often require small incisions for implantation and are prone to affecting the high-weight-bearing cartilage of the donor site. The depth of the defect area should also be analyzed. Most defects do not show significant bone loss. In these cases, an 8mm standard harvesting cannula depth is sufficient to fill the defect area. However, some defects (especially those in chondritis dissecans) show significant bone loss and must be addressed. This can be achieved through a single surgery with bone grafting at the defect site, followed by cartilage transplantation, or by using harvesting cannulas of varying depths to obtain longer emboli, placing the graft so that its cartilage surface is flush with the surrounding cartilage surface. However, this may expose the cancellous bone beneath the emboli at the base of the bone depression.
During transplantation, assessment of the shape of the articular cartilage at both the donor and recipient sites is also important to ensure the best possible match between the cartilage surfaces. For large defects, multiple emboli can effectively reconstruct the original shape of the condyle. While smaller emboli can achieve better shape reconstruction, the reduced strength and stability of the transplanted emboli offset this benefit and increase the number of surgical steps.
- Recommended news
- 【General Surgery Laparoscopy】Cholecystectomy
- Surgery Steps of Hysteroscopy for Intrauterine Adhesion
- [Laryngoscopy in Otolaryngology] Tonsillectomy
- [Otolaryngology Otoscopic Section] Excision of Cholesteatoma in the External Auditory Canal
- [Otolaryngology Nasal Endoscopy] How to Treat Recurrent Rhinitis